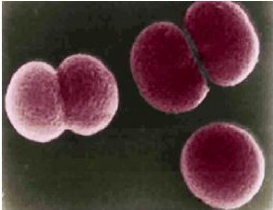
The Hib (Hæmophilus influenzæ type B) influenzae bacterium is a Gram-negative coccobacillus within the normal upper respiratory tract flora. It is spread of is by nasal droplets.
Of the six known types (a to f) of Haemophilus influenzae found in the upper respiratory tract before the introduction of the Hib vaccine, nearly all were of the ‘b capsular’ type.
Incubation period
2 days.
Features
The infection causes an acute illness with fever, vomiting and lethargy, which are symptoms of meningitis. It can also cause epiglottitis and cellulitis.
Complications
About 1 in 20 meningitis patients die and 1 in 4 survive with permanent brain or nerve damage. About 1 in 100 patients with epiglottitis dies.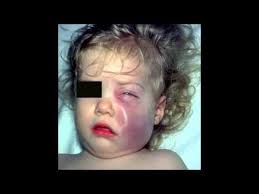
Notification
Suspected and confirmed cases must be notified by telephone immediately and in writing within five days.
History
Before the introduction of the Hib vaccine there were at least 500 cases (10-15 deaths) of Hib disease in children under six, every year – most in children under 18 months of age. Hib was the most common cause of bacterial meningitis in children. Since the introduction of the vaccine to Australia in 1993, there has been a 90% reduction in notified cases.
An account of significant events in Hib immunisation practice in Australia is provided at the NCIRS site.
Vaccine Information.
The Hib vaccine, conjugated to a variety of carrier proteins, is usually given to infants at 2, 4, 6 and 12 months of age, and is very effective. It is part of the National Vaccine Schedule, detailed on the Dept of Health and Ageing web site.
The Hib vaccine is given, by IM injection, either
(a) alone (Hiberix GSK or Liquid PedvaxHIB CSL/Merck),
(b) with hepatitis B vaccine (Comvax CSL/Merck) or
(c) with DTPa, hep B and IPV (Infanrix Hexa GSK). (See ‘Diphtheria’).
Three or four doses are advised according to circumstances, usually in combination with hepatitis B and IPV antigens as in Infanrix Hexa.
About 5% have discomfort or local inflammation; 2% have fever.